Key Notes for Class 11 Chemistry. These notes will provide you overview of the chapters and important points to remember.
Chemistry are covered and are as follow :
UNIT 1: Basic Concept of Chemistry
UNIT 2: Structure of Atom
UNIT 3: Classification of Elements
UNIT 4: Chemical Bonding and Molecular Structure
UNIT 5: States of Matter
UNIT 6: Thermodynamics
UNIT 7: Equilibrium
UNIT 8: Redox Reactions
UNIT 9: The s- Block Element
UNIT 10: The p-block elements
UNIT 11: Organic chemistry
UNIT 12: Hydrocarbon
UNIT 13: Environmental Chemistry
Chapter 1: Some Basic Concepts of Chemistry
* Definition of chemistry and its branches
* States of matter and their properties
* Physical and chemical changes
* Laws of chemical combination: Law of conservation of mass, Law of constant composition, Law of multiple proportions, Law of reciprocal proportions
* Dalton's atomic theory
* Mole concept and Avogadro's number
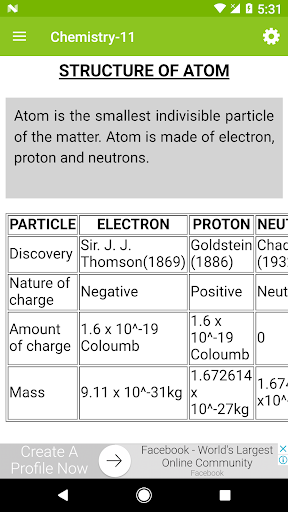
Chapter 2: Structure of Atom
* Discovery of subatomic particles: Electron, proton, and neutron
* Atomic models: Rutherford's model, Bohr's model, and Quantum mechanical model
* Quantum numbers and their significance
* Electronic configuration of elements
Chapter 3: Classification of Elements and Periodicity
* Mendeleev's periodic table and its modern form
* Periodic trends: Atomic radius, ionization energy, electron affinity, electronegativity
* Types of elements: Metals, non-metals, metalloids
* Classification of elements into groups and periods
Chapter 4: Chemical Bonding and Molecular Structure
* Types of chemical bonds: Ionic bond, covalent bond, coordinate bond
* Lewis dot structures and VSEPR theory
* Hybridization of atomic orbitals
* Molecular geometry and polarity
Chapter 5: States of Matter
* Gaseous state: Ideal gas equation, kinetic molecular theory
* Liquid state: Intermolecular forces, viscosity, surface tension
* Solid state: Crystal structures, lattice energy
Chapter 6: Thermodynamics
* Laws of thermodynamics: Zeroth law, First law, Second law, Third law
* Enthalpy, entropy, and free energy
* Equilibrium constants and Le Chatelier's principle
Chapter 7: Equilibrium
* Chemical equilibrium and its characteristics
* Equilibrium constant and its applications
* Factors affecting equilibrium: Temperature, pressure, concentration
Chapter 8: Redox Reactions
* Oxidation and reduction reactions
* Balancing redox reactions using half-reactions
* Electrochemical cells: Galvanic cells and electrolytic cells
Chapter 9: Hydrogen
* Properties and reactions of hydrogen
* Preparation of hydrogen
* Uses of hydrogen
Chapter 10: s-Block Elements
* General properties of Group 1 and Group 2 elements
* Alkali metals: Sodium, potassium
* Alkaline earth metals: Calcium, magnesium
Chapter 11: p-Block Elements
* General properties of Group 13 to Group 18 elements
* Boron and its compounds
* Carbon and its allotropes
* Nitrogen and its compounds
* Oxygen and its compounds
Chapter 12: Organic Chemistry
* Introduction to organic chemistry
* Nomenclature of organic compounds
* Functional groups and their reactions
* Alkanes, alkenes, and alkynes
Chapter 13: Environmental Chemistry
* Environmental pollution and its sources
* Air pollution, water pollution, soil pollution
* Green chemistry and sustainable development
Key Notes for Class 11 Chemistry. These notes will provide you overview of the chapters and important points to remember.
Chemistry are covered and are as follow :
UNIT 1: Basic Concept of Chemistry
UNIT 2: Structure of Atom
UNIT 3: Classification of Elements
UNIT 4: Chemical Bonding and Molecular Structure
UNIT 5: States of Matter
UNIT 6: Thermodynamics
UNIT 7: Equilibrium
UNIT 8: Redox Reactions
UNIT 9: The s- Block Element
UNIT 10: The p-block elements
UNIT 11: Organic chemistry
UNIT 12: Hydrocarbon
UNIT 13: Environmental Chemistry
Chapter 1: Some Basic Concepts of Chemistry
* Definition of chemistry and its branches
* States of matter and their properties
* Physical and chemical changes
* Laws of chemical combination: Law of conservation of mass, Law of constant composition, Law of multiple proportions, Law of reciprocal proportions
* Dalton's atomic theory
* Mole concept and Avogadro's number
Chapter 2: Structure of Atom
* Discovery of subatomic particles: Electron, proton, and neutron
* Atomic models: Rutherford's model, Bohr's model, and Quantum mechanical model
* Quantum numbers and their significance
* Electronic configuration of elements
Chapter 3: Classification of Elements and Periodicity
* Mendeleev's periodic table and its modern form
* Periodic trends: Atomic radius, ionization energy, electron affinity, electronegativity
* Types of elements: Metals, non-metals, metalloids
* Classification of elements into groups and periods
Chapter 4: Chemical Bonding and Molecular Structure
* Types of chemical bonds: Ionic bond, covalent bond, coordinate bond
* Lewis dot structures and VSEPR theory
* Hybridization of atomic orbitals
* Molecular geometry and polarity
Chapter 5: States of Matter
* Gaseous state: Ideal gas equation, kinetic molecular theory
* Liquid state: Intermolecular forces, viscosity, surface tension
* Solid state: Crystal structures, lattice energy
Chapter 6: Thermodynamics
* Laws of thermodynamics: Zeroth law, First law, Second law, Third law
* Enthalpy, entropy, and free energy
* Equilibrium constants and Le Chatelier's principle
Chapter 7: Equilibrium
* Chemical equilibrium and its characteristics
* Equilibrium constant and its applications
* Factors affecting equilibrium: Temperature, pressure, concentration
Chapter 8: Redox Reactions
* Oxidation and reduction reactions
* Balancing redox reactions using half-reactions
* Electrochemical cells: Galvanic cells and electrolytic cells
Chapter 9: Hydrogen
* Properties and reactions of hydrogen
* Preparation of hydrogen
* Uses of hydrogen
Chapter 10: s-Block Elements
* General properties of Group 1 and Group 2 elements
* Alkali metals: Sodium, potassium
* Alkaline earth metals: Calcium, magnesium
Chapter 11: p-Block Elements
* General properties of Group 13 to Group 18 elements
* Boron and its compounds
* Carbon and its allotropes
* Nitrogen and its compounds
* Oxygen and its compounds
Chapter 12: Organic Chemistry
* Introduction to organic chemistry
* Nomenclature of organic compounds
* Functional groups and their reactions
* Alkanes, alkenes, and alkynes
Chapter 13: Environmental Chemistry
* Environmental pollution and its sources
* Air pollution, water pollution, soil pollution
* Green chemistry and sustainable development